| dc.contributor.advisor | Steigen, Sonja E. | |
| dc.contributor.author | Wirsing, Anna Maria | |
| dc.contributor.author | Ervik, Ida Korsnes | |
| dc.contributor.author | Seppola, Marit | |
| dc.contributor.author | Uhlin-Hansen, Lars | |
| dc.contributor.author | Steigen, Sonja Eriksson | |
| dc.contributor.author | Hadler-Olsen, Elin | |
| dc.date.accessioned | 2019-11-01T08:54:15Z | |
| dc.date.available | 2019-11-01T08:54:15Z | |
| dc.date.issued | 2017-10-31 | |
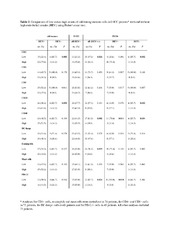
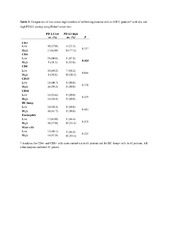
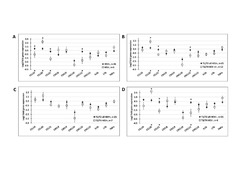
| dc.description.abstract | Oral squamous cell carcinomas (OSCC) are associated with a poor prognosis, which may be partly due to functional impairment of the immune response. Lymphocyte recruitment to the tumor site is facilitated by high-endothelial venules (HEV), whereas expression of programmed-death ligand 1 (PD-L1) can impair T cell function. Thus, we hypothesize that these factors are important in shaping the immune response in OSCC. In the present study, we characterized the immune infiltrate in formalin-fixed, paraffin-embedded tumor samples from 75 OSCC patients. We used immunohistochemistry to determine the distribution of immune cell subsets, HEV and PD-L1, as well as quantitative real-time polymerase chain reaction to assess the expression of inflammatory cytokines and chemokines associated with lymphocyte trafficking. Finally, we calculated correlations between the presence of immune cell subsets, the gene expression patterns, HEV, PD-L1 and the clinicopathological parameters including patient survival. The presence of HEV correlated with increased number of CD3+ T cells and CD20+ B cells, higher levels of the chemokines CXCL12 and CCL21, and lower levels of CCL20, irrespective of the tumors’ T-stage. In univariate analysis, high levels of CD20+ B cells and CD68+ macrophages, positive HEV-status, and low T- and N-stages predicted longer patient survival. However, only the presence of HEV and a low T-stage were independent positive prognosticators. This indicates that HEV are important mediators and a convenient marker of an antitumor immune response in OSCC. Our findings support HEV as a potential immunomodulatory target in OSCC. PD-L1 staining in tumor cells correlated with lower T-stage, increased infiltration of CD4+ cells and higher expression of several inflammation-related cytokines. Thus, OSCC tumors rich in CD4+ cells may preferentially respond to PD-1/PD-L1 blockade therapy. | en_US |
| dc.identifier.uri | https://hdl.handle.net/10037/16559 | |
| dc.language.iso | eng | en_US |
| dc.publisher | UiT Norges arktiske universitet | en_US |
| dc.publisher | UiT The Arctic University of Norway | en_US |
| dc.rights.accessRights | openAccess | en_US |
| dc.rights.holder | Copyright 2017 The Author(s) | |
| dc.rights.uri | https://creativecommons.org/licenses/by-nc-sa/3.0 | en_US |
| dc.rights | Attribution-NonCommercial-ShareAlike 3.0 Unported (CC BY-NC-SA 3.0) | en_US |
| dc.subject.courseID | MED-3910 | |
| dc.subject | VDP::Medical disciplines: 700::Basic medical, dental and veterinary science disciplines: 710::Medical immunology: 716 | en_US |
| dc.subject | VDP::Medisinske Fag: 700::Basale medisinske, odontologiske og veterinærmedisinske fag: 710::Medisinsk immunologi: 716 | en_US |
| dc.subject | VDP::Medical disciplines: 700::Basic medical, dental and veterinary science disciplines: 710::General pathology, anatomical pathology: 719 | en_US |
| dc.subject | VDP::Medisinske Fag: 700::Basale medisinske, odontologiske og veterinærmedisinske fag: 710::Generell patologi, patologisk anatomi: 719 | en_US |
| dc.subject | VDP::Medical disciplines: 700::Clinical dentistry disciplines: 830::Oral medicine: 835 | en_US |
| dc.subject | VDP::Medisinske Fag: 700::Klinisk odontologiske fag: 830::Oral kirurgi: 835 | en_US |
| dc.title | Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carsinoma | en_US |
| dc.type | Master thesis | en_US |
| dc.type | Mastergradsoppgave | en_US |


 English
English norsk
norsk